By Andrew Heck
Editor’s note: This article has been corrected regarding the xenotransplantation of a pig kidney, which was performed on a brain-dead human.

When science fiction becomes science fact, heads quickly turn. Such is the case with gene editing – an emerging suite of technologies enabling precise changes to the DNA of a cell or animal.
While the discovery of DNA in the 1950s heralded a new age for biotechnology, the field really began to take off after the 1990s, thanks to successive breakthroughs in genetic engineering.
Fast-forward to the 2020s, and gene editing technologies are now taking centre stage, with more than 35,000 scientific papers published on the subject since 2013, including 7,000 alone in 2022. More than 100 of those published last year were related to pigs, specifically in the field of biomedical science.
Just prior to the start of the 2023 Banff Pork Seminar, Swine Innovation Porc (SIP) hosted three researchers to present their knowledge on the subject, including Ray Lu from the Department of Molecular and Cellular Biology at the University of Guelph, Vilceu Bordignon from the Department of Animal Science at McGill University, and Stuart Smyth from the Department of Agricultural and Resource Economics at the University of Saskatchewan.
The researchers each spoke on different aspects of gene editing. For Lu, the possibility of using gene editing as a solution to the wild boar problem; for Bordignon, an exploration of where the technology has been successfully deployed in various aspects of our world; and, for Smyth, the state of the technology’s public acceptance.
“Our goal in presenting this information is not about being ‘for’ or ‘against’ gene editing,” said Arno Schober, Chair, SIP. “It’s about bringing together farmers and researchers to have discussions about how we can prepare for the future and what role it could play in our industry.”
To begin to understand the basics of gene editing, it is important to establish what it is. ‘CRISPR/Cas’ gene editing is a precision genetic toolbox that singles out individual nucleic acids – the building blocks of DNA. This allows for the creation of new gene sequences at the same level as nature, in principle. Genetic variations exist naturally between organisms but also between cells within an organism, and most of these differences play no role in ‘phenotypes,’ which are the visible expressions of genetic variation, such as what makes brown hair, brown, and blonde hair, blonde.
Comparing gene editing to genetic modification or ‘GMO,’ the major difference is that gene editing relies on existing genetic material to perform edits, rather than introducing new genetic material – typically from entirely different organisms – which is the case with GMO.
At the higher level, more traditionally, genetic variation within livestock has been brought about through selective breeding strategies. These have, for a long time, led to the favourable genetic advancements in pigs that we can all appreciate. But can isolating, slicing and replacing strands of DNA achieve results in a similar fashion, with greater speed, efficiency and effectiveness? Certainly, and science is only beginning to scratch the surface.
A radically different approach to wild boar

On the Canadian prairies, in parts of Ontario and across the southeastern U.S., wild boar – sometimes referred to as ‘feral swine’ or by other terms – are a highly destructive invasive species. Controlling wild boar populations has proven incredibly difficult or impossible, in some regions. Hunting causes their groups to scatter and infest new areas, leaving the most credible solution, at present, to be tracking, trapping and culling whole groups, called ‘sounders.’
But what if there was a proactive way to manage the wild boar population? Ray Lu believes gene editing could help reduce the harm they cause in two ways: one, by making them resistant to the African Swine Fever (ASF) virus, or, two, by limiting their ability to replenish their numbers so quickly.
The first option is called ‘population replacement,’ which refers to editing an organism’s genome so the specific gene that transmits a certain pathogen is altered in a way that does not allow for transmission. With successive generations of reproduction, the altered gene becomes more prevalent, eventually snuffing out any virus-carrying potential. The second option is called ‘population suppression,’ which acts as a method of generating female infertility to reduce wild boar numbers outright.
In 2015, Pig Improvement Company (PIC) began to develop a gene-edited pig resistant to porcine reproductive and respiratory syndrome (PRRS). Currently, the company is working toward making the pig commercially available. There has been a great deal of enthusiasm around the PRRS-resistant pig, and some have wondered if the approach can be applied to other swine viruses.
“For ASF resistance, it is more complex,” said Lu. “This virus is very stable in the environment.”
ASF is presently less widespread than certain other swine viruses, including PRRS, but its existence anywhere is much more concerning for the pork value chain, collectively, along with Foot-and-Mouth Disease (FMD), which affects swine and several species of ruminants. PRRS continues to take a massive toll on swine herds around the world, and recovery from PRRS is costly for individually affected farms; however, the discovery of an ASF or FMD case in Canadian wild boar or a domestic pig herd would close our borders to the international markets into which around 70 per cent of all pigs and pork produced and processed in our country are sold. Given the relationship between Canadian and U.S. markets when it comes to pricing pigs and pork, a case of ASF or FMD discovered in the U.S. could be equally devastating for Canadian exports of live animals or pork and beef products.
While the agri-food industry certainly has a vested interest in controlling wild boar and diseases they carry, other disease issues have a broader, global impact on humans. Mosquito-borne viruses like dengue, malaria, Zika and West Nile kill upwards of one million people annually, with the vast majority of cases and deaths affecting people in Africa, especially young children. As such, the scientific community has been actively pursing gene editing solutions to combat these viruses, demonstrating that the real potential for positive improvements in this area goes well beyond the hype. Suffice to say, the world is ready for any credible strategy that lowers the toll of insect- or animal-borne disease transmission, which is a promising inclination for everyone.
Where gene editing is already finding success

Vilceu Bordignon believes advancements in gene editing can have mutual benefits for human medicine and livestock production, while also recognizing the pitfalls.
“Not all genes can be edited,” said Bordignon, referring to genes that are fundamental to an organism’s existence. “What we do in the lab isn’t ‘natural,’ but it’s pretty close to what nature does.”
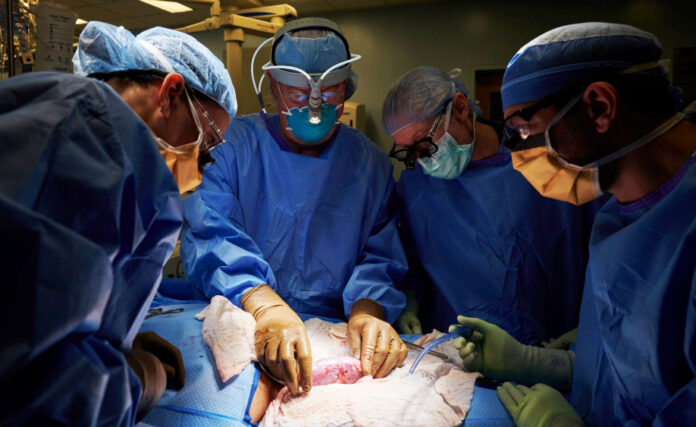
Bordignon provided several examples of how the technology is already being deployed, including ‘xenotransplantation,’ which is the transfer of living cells, tissues or organs from animals to humans. Notable instances include a pig-to-human kidney transplant, in late 2021, and a pig-to-human heart transplant, in early 2022. Both examples were taken from pigs that had 10 gene edits applied.
In the case of the heart transplant, the recipient, unfortunately, passed away just two months following the procedure. The recipient was a 57-year-old male with terminal heart disease. Had nothing been done, his fate may have been the same. But thanks to his willingness to participate in the experimental procedure, his contribution and the work of the surgeons who performed the transplant will long be remembered as a necessary step toward meaningful progress. Such examples exist in many scientific fields throughout history, and their value cannot be overstated.
In livestock production, gene editing is more familiar to the bovine industry. It has already been used to produce hornless dairy cattle with genome-edited cells, and within the past year, the U.S. Food and Drug Administration (FDA) has approved beef from gene-edited cattle for human consumption.
“Gene editing is much more than cutting genes with scissors; it’s more like a Swiss Army Knife,” said Bordignon. “The CRISPR/Cas system allows for all kinds of modifications and combinations.”
Piglet castration is a contested practice within production, along with tail-docking. While it makes practical sense, the animal welfare component is constantly brought to light. The main goal of castration is to remove the testes to prevent male pigs from reaching sexual maturity, to avoid ‘boar taint’ in meat. A novel approach could be to use gene editing to delay puberty beyond market age – so it is never fully reached – or generate sex reversals in utero by preventing the expression of the genes that present the male phenotype, resulting in an all-female piglet litter.
No matter where gene editing in livestock is headed, the possibilities cannot be denied, while the full scale of unknowns surrounding the technology warrants further research and dialogue across the agri-food industry and society.
Getting public buy-in on gene editing

Looking at the history of innovation and science within the public consciousness, Stuart Smyth presented some alternative examples of how, over time, acceptance continues to grow.
‘Mutation breeding’ has existed since the 1920s, when researchers zapped crops like corn and barley with X-rays to find out if radiation could be used to create beneficial genetic variations. It worked, and it has been instrumental in advancing crop science ever since. But imagine the reaction of someone a century ago after being told that the same corn and barley their ancestors had grown for years could be drastically improved through advances in biotechnology – like magic, to the untrained eye. Are we, today, going through a similar shift with gene editing?
“Messaging around this issue is just as important as the science,” said Smyth. “Those people who are fighting against science and innovation in agriculture are appealing to emotion. If the messaging doesn’t appeal to emotion, the public will reject it.”
As of 2022, Health Canada no longer requires additional risk assessments on gene-edited crops; however, we still have no regulatory framework for gene-edited animals.
“The pace of innovation exceeds that of regulation, causing lengthy delays,” said Smyth.
Public perception in Canada on gene editing is currently split. In 2018, two-thirds of Canadians were opposed. In 2020, that decreased to just over half, with many remaining uncertain. Yet, it is becoming abundantly clear that the Canadian pork sector has an opportunity to prevent zoonotic diseases and overcome potential trade barriers by using gene editing.
Trade protectionism is increasing in the world, and when it comes to meat and plant products crossing borders, buyers are becoming even bigger skeptics on the disease front. If gene editing can create a closer-to-guaranteed solution to disease issues, in addition to farm biosecurity and in-plant food handling practices, Canada’s important trade relationships may be easier to preserve with airtight confidence.
While keeping borders open is the goal of free trade, the biggest challenge, it seems, may be to open minds.
Benefits of gene editing coming into focus

Wherever you find yourself on the spectrum of opinions about gene editing, there is little doubt, given the growing body of research interest, that the subject is poised to change many aspects of our world, including the pork sector.
“We are a science-based industry; that’s a big part of the success of Canadian agriculture,” said Daniel Ramage, Executive Director, SIP. “It’s how we do more with less, and it’s how we produce safe food efficiently. Research is a foundation to that.”
If gene editing can lead to better pork production, safer food or enhanced human medicine, it is increasingly likely you could soon come across gene-edited technologies in a barn, grocery store or hospital near you. As time goes on, however, wrinkles in the social fabric caused by gene editing might prove even more stubborn than the brilliant minds using gene editing to achieve the goals of scientific progress.